|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Several basic elements of neurosurgical/neuroanesthetic management are recurrent and should be discussed and agreed on with the surgical team at the outset of every neurosurgical procedure. The list will vary with the procedure and may include the intended surgical position and requisite positioning aids; intentions with respect to the use of steroids, diuretics, anticonvulsants, and antibiotics; the surgeon's perception of the "tightness" of the intracranial space and the remaining intracranial compliance reserve; appropriate objectives for the management of blood pressure, carbon dioxide tension, and body temperature; anticipated blood loss; the intended use of neurophysiologic monitoring (which may impose constraints on the use of anesthetics or muscle relaxants, or both); and occasionally, the perceived risk of air embolization. The considerations driving the decisions made about these issues are presented in this section. One additional recurrent issue, brain protection, is discussed briefly in the section "Aneurysms and Arteriovenous Malformations" and in detail in Chapter 21 .
The necessity of preventing increases in intracranial pressure (ICP) or reducing an ICP that is already elevated is recurrent in neuroanesthesia. When the cranium is closed, the objective is to maintain adequate cerebral perfusion pressure (CPP) (CPP = mean arterial pressure [MAP] − ICP) and prevent the herniation of brain tissue between intracranial compartments or through the foramen magnum ( Fig. 53-1 ). When the cranium is open, the issue may be one of providing relaxation of the intracranial contents to facilitate surgical access or, in extreme circumstances, reverse the process of brain herniation through a craniotomy (see Fig. 53-1 ). The principles that apply are similar whether the cranium is open or closed.
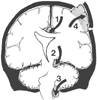 Figure 53-1
Schematic representation of various herniation pathways:
1, subfalcine; 2, uncal (transtentorial); 3, cerebellar; and 4, transcalvarial.
(Reprinted, by permission, from Fishman RA: Brain edema. N Engl J Med
293:706, 1975.)
Figure 53-1
Schematic representation of various herniation pathways:
1, subfalcine; 2, uncal (transtentorial); 3, cerebellar; and 4, transcalvarial.
(Reprinted, by permission, from Fishman RA: Brain edema. N Engl J Med
293:706, 1975.)
The various clinical indicators of increased ICP include headache (particularly a postural headache that awakens the patient at night), nausea and vomiting, blurred vision, somnolence, and papilledema. Suggestive findings on computed tomography (CT) include midline shift, obliteration of the basal cisterns, loss of sulci, ventricular effacement (or enlarged ventricles in the event of hydrocephalus), and edema. Edema appears on a CT scan as a region of hypodensity. The basal cisterns appear on CT as a black (fluid) halo around the upper end of the brainstem ( Fig. 53-2 ). They include the interpeduncular cistern, which lies
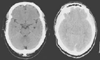 Figure 53-2
Computed tomography scan depicting normal (left)
and compressed (right) basal cisterns. The basal,
or perimesencephalic, cerebrospinal fluid space consists of the interpeduncular cistern
(anterior), the ambient cisterns (lateral), and the quadrigeminal cisterns (posterior).
In the right panel, in a patient with diffuse cerebral
swelling (as a result of sagittal sinus thrombosis), the cisterns have been obliterated.
(Courtesy of Ivan Petrovitch, M.D.)
Figure 53-2
Computed tomography scan depicting normal (left)
and compressed (right) basal cisterns. The basal,
or perimesencephalic, cerebrospinal fluid space consists of the interpeduncular cistern
(anterior), the ambient cisterns (lateral), and the quadrigeminal cisterns (posterior).
In the right panel, in a patient with diffuse cerebral
swelling (as a result of sagittal sinus thrombosis), the cisterns have been obliterated.
(Courtesy of Ivan Petrovitch, M.D.)
Figure 53-3 presents the pressure-volume relationship of the intracranial space. The plateau phase occurring at low volumes reveals that the intracranial space is not a completely closed one and that there is some compensatory latitude. Compensation is accomplished principally by the translocation of cerebrospinal fluid (CSF) and venous blood to the spinal CSF space and the extracranial veins, respectively. Ultimately, when the compensatory potential is exhausted, even tiny increments in volume of the intracranial contents can result in substantial increases in ICP. These increases have the potential to result in either herniation of brain tissue from one compartment to another (or into the surgical field) (see Fig. 53-1 ), with resultant mechanical injury to brain tissue, or reduction in perfusion pressure with a concomitant ischemic injury.
Several variables can interact to cause or aggravate intracranial hypertension ( Fig. 53-4 ). For clinicians faced with the problem of managing increased ICP, the objective is, broadly speaking, to reduce the volume of the intracranial contents. For mnemonic purposes, when developing a clinical approach, the clinician can divide the intracranial space into four subcompartments ( Table 53-1 ): cells (including neurons, glia, tumors, and extravasated collections of blood), fluid (intracellular and extracellular), CSF, and blood. Again for mnemonic purposes, the blood compartment can be subdivided into venous and arterial components. It is this last compartment, the blood compartment, that is most amenable to rapid manipulation by the clinician, and accordingly, it is the compartment to which the greatest level of attention is ultimately directed.
 Figure 53-3
Intracranial pressure-volume relationship. The horizontal
portion of the curve indicates that initially there is some latitude for compensation
in the face of an expanding intracranial lesion. This compensation is accomplished
largely by displacement of cerebrospinal fluid (CSF) and venous blood from the intracranial
to the extracranial spaces. Once the compensatory latitudes are exhausted, small
increments in volume result in large increases in intracranial pressure with the
associated hazards of herniation or decreased cerebral perfusion pressure (CPP) resulting
in ischemia.
Figure 53-3
Intracranial pressure-volume relationship. The horizontal
portion of the curve indicates that initially there is some latitude for compensation
in the face of an expanding intracranial lesion. This compensation is accomplished
largely by displacement of cerebrospinal fluid (CSF) and venous blood from the intracranial
to the extracranial spaces. Once the compensatory latitudes are exhausted, small
increments in volume result in large increases in intracranial pressure with the
associated hazards of herniation or decreased cerebral perfusion pressure (CPP) resulting
in ischemia.
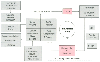 Figure 53-4
Pathophysiology of intracranial hypertension. The figure
depicts the manner in which increases in the volumes of any or all of the four intracranial
compartments, blood, cerebrospinal fluid (CSF), fluid (interstitial or intracellular),
and cells (four-part rectangle) result in increases in intracranial pressure and
eventual neurologic damage. Elements that are potentially under control of the anesthesiologist
are indicated by asterisks. (Control of CSF volume
requires the presence of a ventriculostomy catheter.) The herniation pathways are
depicted in Figure 53-1
.
Figure 53-4
Pathophysiology of intracranial hypertension. The figure
depicts the manner in which increases in the volumes of any or all of the four intracranial
compartments, blood, cerebrospinal fluid (CSF), fluid (interstitial or intracellular),
and cells (four-part rectangle) result in increases in intracranial pressure and
eventual neurologic damage. Elements that are potentially under control of the anesthesiologist
are indicated by asterisks. (Control of CSF volume
requires the presence of a ventriculostomy catheter.) The herniation pathways are
depicted in Figure 53-1
.
| Compartment | Volume Control Methods |
|---|---|
| 1. Cells (including neurons, glia, tumors, and extravasated blood) | Surgical removal |
| 2. Fluid (intracellular and extracellular) | Diuretics |
|
|
Steroids (principally tumors) |
| 3. Cerebrospinal fluid | Drainage |
| 4. Blood |
|
| Arterial side | Decrease cerebral blood flow |
| Venous side | Improve cerebral venous drainage |
We suggest giving first consideration to the venous side of the circulation. It is largely a passive compartment that is frequently overlooked. Passive though it is, engorgement of this compartment is a common cause of increased ICP or poor conditions in the surgical field ( Fig. 53-5 ). A head-up posture to ensure good venous drainage is the norm in neurosurgical anesthesia and critical care. Obstruction of cerebral venous drainage by extremes of head position or circumferential pressure (Philadelphia collars, endotracheal tube ties) should be avoided. Phenomena occurring downstream from the venous structures of the neck may also be relevant. Anything that causes increased intrathoracic pressure can result in obstruction of cerebral venous drainage. A variety of
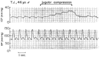 Figure 53-5
Effect of obstruction of cerebral venous outflow on intracranial
pressure (ICP) in a patient with intracerebral hematoma. Bilateral jugular compression
was applied briefly to verify the function of a newly placed ventriculostomy. The
ICP response illustrates the importance of maintaining unobstructed cerebral venous
drainage.
Figure 53-5
Effect of obstruction of cerebral venous outflow on intracranial
pressure (ICP) in a patient with intracerebral hematoma. Bilateral jugular compression
was applied briefly to verify the function of a newly placed ventriculostomy. The
ICP response illustrates the importance of maintaining unobstructed cerebral venous
drainage.
Thereafter, the anesthesiologist should consider the arterial side of the circulation. Attention to the effect of anesthetic drugs and techniques on cerebral blood flow (CBF) (see Chapter 21 ) is an established part of neuroanesthesia. Such attention is relevant because in general, increases in CBF are associated with increases in cerebral blood volume (CBV).[1] [2] [3] The notable exception to this rule occurs in the context of cerebral ischemia caused by hypotension or vessel occlusion, when CBV may increase as the cerebral vasculature dilates in response to a sudden reduction in CBF. However, the relationship generally applies, and attention to the control of CBF is relevant in situations in which volume compensation mechanisms are exhausted or ICP is already increased. The general approach is to select anesthetics and control the physiologic parameters in a manner that avoids unnecessary increases in CBF. The parameters that influence CBF are listed in Table 53-2 and are discussed in Chapter 21 .
The question of which anesthetics are appropriate, especially in the context of unstable ICP, arises often. Chapter 21 provides relevant information in detail, and only broad generalizations are made here.
In general, intravenous anesthetic, analgesic, and sedative drugs are associated with parallel reductions in CBF and cerebral metabolic rate (CMR) and will not have adverse effects on ICP. Ketamine, given in large doses to patients with a generally normal level of consciousness before anesthesia, may be the exception. It appears that for the most part, autoregulation and CO2 responsiveness are preserved during the administration of all intravenous drugs ( Chapter 21 ).
By contrast, all of the volatile anesthetics cause dose-dependent cerebral vasodilation. The order of vasodilating potency is approximately halothane ≫ enflurane > isoflurane > desflurane > sevoflurane. As noted in Chapter 21 , the CBF differences among isoflurane, desflurane, and sevoflurane are probably not significant to the clinician. The net CBF effect of introducing a volatile anesthetic will depend on the interaction of several factors: the concentration of the anesthetic, the extent of previous CMR depression, simultaneous blood pressure changes acting in conjunction with previous or anesthetic-induced autoregulation abnormalities, and simultaneous changes in PaCO2 acting in conjunction with any disease-related impairment in CO2 responsiveness.
Nitrous oxide is also a cerebral vasodilator, the CBF effect of
which is greatest when it is administered as a sole anesthetic, least when it is
administered against a background of narcotics, propofol, or benzodiazepines, and
intermediate when administered in conjunction
| PaO2 |
| PaCO2 |
| Cerebral metabolic rate |
| Arousal/pain |
| Seizures |
| Temperature |
| Anesthetics |
| Blood pressure/status of autoregulation |
| Vasoactive agents |
| Anesthetics |
| Pressors |
| Inotropes |
| Vasodilators |
| Blood viscosity |
| Neurogenic pathways (intra- and extra-axial) |
Muscle relaxants ( Chapter 13 and Chapter 21 ) that have the potential to release histamine (curare, metocurine, mivacurium, atracurium) should be given in small, divided doses. Although succinylcholine has been associated with increases in ICP, these increases are small and transient. Moreover, the increases can be blocked by a preceding dose of metocurine, 0.03 mg/kg,[5] and in at least some instances, are not evident in patients with common emergency neurosurgical conditions (head injury, subarachnoid hemorrhage [SAH]).[6] Accordingly, in a clinical situation that calls for rapid relaxation for the purpose of controlling or protecting the airway, succinylcholine in conjunction with proper management of the airway and MAP is reasonable.
From the material just presented and the preceding discussion of cerebral physiology in Chapter 21 , a systematic clinical approach should follow readily. A schema for approaching the problem of an acute increase in ICP or acute deterioration in conditions in the surgical field is presented in Table 53-3 .
If the problem has not resolved satisfactorily after following the approach in Table 53-3 , what then? Table 53-4 presents the options.
CSF drainage was discussed earlier. The use of additional osmotic
diuretics is theoretically limited by an upper acceptable osmolarity limit of approximately
320 mOsm/L. However, in extremis, the use is frequently empirical, and repeated
doses (e.g., 12.5 g) are administered until a clinical response is no longer observed.
Barbiturates have long been used most to induce a reduction in CMR, with the objective
of causing a coupled reduction in CBF and thereby CBV. Propofol is gaining popularity
for this application. Note, however, that although the use of barbiturates is supported
by intensive care unit (ICU) experience demonstrating efficacy in control[7]
(if not outcome) of ICP, no such experience has been accumulated for propofol. Furthermore,
a frequently fatal syndrome of metabolic acidosis and rhabdomyolysis has recently
been recognized in patients who have received prolonged propofol infusions in the
ICU setting.[8]
[9]
[10]
MAP reduction will occasionally
| 1. Are the relevant pressures controlled? |
| Jugular venous pressure |
| Extreme head rotation or neck flexion? |
| Direct jugular compression? |
| Head-up posture? |
| Airway pressure |
| Airway obstruction? |
| Bronchospasm? |
| Straining, coughing, adequately relaxed? |
| Pneumothorax? |
| Partial pressure of CO2 and O2 (PaCO2 , PaO2 ) |
| Arterial pressure |
| 2. Is the metabolic rate controlled? |
| Pain/arousal? |
| Seizures? |
| 3. Are any potential vasodilators in use? |
| N2 O, volatile anesthetics, nitroprusside, calcium channel blockers |
| 4. Are there any unrecognized mass lesions? |
| Blood, air ± N2 O |
|
|
|
|
|
|
|
|
|
|
|
|
|