|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The term balanced anesthesia was introduced by Lundy in 1926. Lundy suggested that a balance of agents and techniques be used to produce the different components of anesthesia (i.e., analgesia, amnesia, muscle relaxation, and abolition of autonomic reflexes with maintenance of homeostasis). Anesthesia with a single agent can require doses that produce excessive hemodynamic depression.[365] The inclusion of an opioid as a component of balanced anesthesia can reduce preoperative pain and anxiety, decrease somatic and autonomic responses to airway manipulations, improve hemodynamic stability, lower requirements for inhaled anesthetics, and provide immediate postoperative analgesia. [366] Although the intent of combining opioids with sedative-hypnotics and/or volatile anesthetics is to produce anesthetic conditions with stable hemodynamics prior to, as well as after, noxious stimulation, this ideal is not always achieved.[367] [368] [369]
The administration of an opioid prior to, rather than after, noxious stimulation attenuates physiologic responses. Opioids interact synergistically and markedly reduce the dose of propofol and other sedative-hypnotics required for loss of consciousness and during noxious stimulation such as skin incision.[370] Opioids can ameliorate or eliminate responses to a rapid sequence induction and other noxious stimuli.[371] The heart rate response to laryngoscopy is better controlled with an opioid than with esmolol.[366]
The timing, rate of administration, and dose of supplemental opioid should be tailored to the specific condition of the patient and the expected duration of the operation in order to avoid postoperative pain or respiratory depression. A large dose of any opioid given shortly before the end of surgery is very likely to result in respiratory depression. Analgesic concentrations of opioids,
The ideal opioid would permit rapid titration, successfully prevent unwanted responses to noxious stimuli, require little supplementation, not depress cardiovascular function, permit the return of adequate spontaneous ventilation in a timely manner, and produce residual if not complete postoperative analgesia with minimal side effects.
Alfentanil, and remifentanil provide the greatest ability to titrate opioids rapidly because of their extremely rapid time to onset (1 to 2 minutes) of peak effect. Alfentanil, compared with fentanyl, produces greater decreases in heart rate and blood pressure during induction of anesthesia.[373] [374] Most studies have found that alfentanil results in a more rapid recovery than fentanyl and a decreased need for naloxone post-anesthetically. [373] Compared with alfentanil, sufentanil provides greater hemodynamic stability and requires less supplementation.[375] Meperidine produced the most side effects, including hypotension, tachycardia, and urticaria, especially during anesthetic induction.
Sufentanil, alfentanil, and remifentanil are arguably superior to fentanyl in many respects. Antagonism of opioid action with naloxone for troublesome respiratory depression is required less frequently after alfentanil and sufentanil compared with fentanyl. Pharmacologic antagonism is rarely required after remifentanil administration.
Anesthetic induction is usually achieved by combining a loading dose of fentanyl (2–6 µg/kg) with a sedative-hypnotic, most commonly thiopental or propofol, and a muscle relaxant. Maintenance of anesthesia can be achieved with N2 O (60% to 70%) in O2 , low concentrations of potent inhaled anesthetics, and additional fentanyl (intermittent boluses of 25–50 µg every 15 to 30 minutes or a constant infusion of 0.5–5.0 µg/kg/hour), depending on the depth of anesthesia required versus the intensity and duration of surgery.
Fentanyl plasma concentrations of 1.0 to 2.0 ng/mL provide analgesia, [294] but levels of at least 2 to 3 ng/mL are usually required during surgery if the only inhaled agent is N2 O. In unpremedicated patients anesthetized with a fentanyl infusion and N2 O in O2 , the Cp50 and the Cp50 -BAR (barish autonomic response) for fentanyl at skin incision are 3.26 and 4.17 ng/mL, respectively.[376] The MAC of isoflurane at skin incision can be reduced by 50% and 63% with plasma fentanyl concentrations of 1.67 and 3.0 ng/mL, respectively.[62] Increasing plasma fentanyl concentrations from 3.0 to 10 ng/mL only further reduced the MAC of isoflurane from 63% to 82%. Plasma fentanyl levels associated with adequate analgesia after abdominal surgery have been reported to range fivefold from 0.23 to at least 1.18 ng/mL.[294]
Opioid pharmacokinetics and pharmacodynamics vary considerably among patients. Nevertheless, a balanced technique with fentanyl, titrating the opioid in anticipation of various stimuli and patient responses with pharmacokinetic guidelines in mind, will often result in a stable hemodynamic course and rapid awakening in a relatively pain-free patient. Repeated large doses or continuous infusions of fentanyl at high rates are most likely to result in significant depression of spontaneous ventilation.
Because alfentanil penetrates the brain so rapidly, equilibration of alfentanil between the plasma and the CNS can be achieved while plasma alfentanil levels are relatively high compared with sufentanil and fentanyl. This property explains how low doses (10–30 µg/kg) of alfentanil, administered just before or simultaneously with a sedative-hypnotic, are effective.
Alfentanil (25–50 µg/kg IV), followed by small titrated sleep doses of any sedative-hypnotic (e.g., 50–100 mg sodium thiopental), is usually successful in preventing significant hemodynamic stimulation from laryngoscopy and intubation. Further opioid supplementation can be achieved with an alfentanil infusion (0.5–2.0 µg/kg/minute) or intermittent boluses of alfentanil (5–10 µg/kg) for shorter procedures. In balanced anesthetic techniques in which potent inhaled anesthetics are also employed, relatively low plasma alfentanil concentrations (e.g., 29 ng/mL) can reduce the MAC of isoflurane by approximately 50%.[78] The Cp50 , defined as the concentration needed to obtund responses to stimuli adequately in 50% of patients, is different among various stimuli.[163] It is remarkable that the more intense the stimulus, the greater the variability in the Cp50 ( Fig. 11-27 ).
Alfentanil infusions or repeated doses should be reduced 15 to 30 minutes prior to the end of surgery, in order to avoid problematic residual respiratory depression. The alfentanil plasma concentration that permitted the return of spontaneous ventilation was reported to be 223 ± 13 ng/mL.[163] Although alfentanil infusions should be reduced as necessary during balanced anesthesia, they should not be completely terminated until the operation is nearly finished.
The mean plasma sufentanil concentration reported to be the Cp50 for prevention of hemodynamic responses to laryngoscopy and tracheal intubation was 1.08 ng/mL, with a range of 0.73 to 2.55 ng/mL. Maintenance of anesthesia can be achieved with N2 O (60% to 70%) in O2 and further sufentanil (intermittent boluses, 0.1–0.25 µg/kg, or constant infusion, 0.5–1.5 µg/kg/hour). The Cp50 for sufentanil during skin incision is twice as great as that for intubation in unpremedicated patients (2.08 ± 0.62 ng/mL).[377] Cp50 value ratios at skin incision for sufentanil, fentanyl, and alfentanil in N2 O-O2 anesthesia are approximately 1:2:150 and represent different and probably more accurate potency ratios than those traditionally published based on drug dose. In patients undergoing coronary artery bypass grafting, sufentanil >1.25 ± 0.21 ng/mL reduced isoflurane requirements to less than 0.5% through the operation.[378] Sufentanil levels less than 0.25 ng/mL often allow adequate spontaneous ventilation.[379]
The very short duration of action of remifentanil mandates that an infusion (0.1–1.0 µg/kg/minute) be started prior to or soon after the initial bolus dose to ensure sustained opioid effect. Maintenance infusion rates of
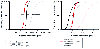 Figure 11-27
Mean alfentanil plasma concentration-effect curves for
the intraoperative period in each surgical group of patients, intubation, skin incision,
and skin closure (with nitrous oxide), and the relationship between the alfentanil
plasma concentration (without nitrous oxide) and the probability of needing naloxone
to restore adequate spontaneous ventilation. The Cp50
and slope of these
curves were determined by averaging the estimates of individual patients. Bars indicate
standard deviation of Cp50
. Cp50
, the concentration needed
to obtund responses to stimuli adequately in 50% of patients. (From Ausems
ME, Hug CC Jr, Stanski DR, Burm AG: Plasma concentrations of alfentanil required
to supplement nitrous oxide anesthesia for general surgery. Anesthesiology 65:362–367,
1986.)
Figure 11-27
Mean alfentanil plasma concentration-effect curves for
the intraoperative period in each surgical group of patients, intubation, skin incision,
and skin closure (with nitrous oxide), and the relationship between the alfentanil
plasma concentration (without nitrous oxide) and the probability of needing naloxone
to restore adequate spontaneous ventilation. The Cp50
and slope of these
curves were determined by averaging the estimates of individual patients. Bars indicate
standard deviation of Cp50
. Cp50
, the concentration needed
to obtund responses to stimuli adequately in 50% of patients. (From Ausems
ME, Hug CC Jr, Stanski DR, Burm AG: Plasma concentrations of alfentanil required
to supplement nitrous oxide anesthesia for general surgery. Anesthesiology 65:362–367,
1986.)
The use of remifentanil allows a rapid (5 to 15 minutes) emergence without postoperative respiratory depression.[381] Infusion rates of 0.1 ± 0.05 µg/kg/minute should permit the return of spontaneous ventilation and responsiveness with maintenance of analgesia within 10 to 15 minutes. A randomized, double-blind, placebo-controlled study demonstrated that the combination of 0.05–0.1 µg/kg/minute remifentanil infusion and a 2-mg midazolam bolus provided effective sedation and analgesia during outpatient surgical procedures performed under local anesthesia.[382] The administration of remifentanil (1 µg/kg) followed by 0.5 µg/kg/minute with propofol and 66% N2 O provided sufficient anesthesia for craniotomy with stable hemodynamics and rapid tracheal extubation.[383]
Associated with emergence from remifentanil anesthesia, the need for alternative analgesic therapies should be anticipated and administered in a timely fashion prior to or immediately upon emergence from anesthesia. It was reported that perioperative administration of morphine (0.15 or 0.25 mg/kg IV) or fentanyl (0.15 mg) did not provide entirely adequate immediate postoperative pain control after remifentanil-based anesthesia in major abdominal surgery.[384] [385] Administration of ketamine (0.15 mg/kg followed by 2 µg/kg/minute) decreased intraoperative remifentanil use during abdominal surgery and postoperative morphine consumption without increasing the incidence of side effects.[386] In children undergoing strabismus surgery, the combination of sevoflurane (2.5%) and remifentanil (1 µg/kg followed by 0.1–0.2 µg/kg/minute) resulted in less frequent postoperative vomiting but higher postoperative pain scores, compared with fentanyl (2 µg/kg followed by 1 µg/kg every 45 minutes).[387]
Postoperative pain relief with low-dose remifentanil infusion has also been achieved successfully. After general anesthesia with propofol (75 µg/kg/minute) and remifentanil (0.5–1.0 µg/kg/minute) for abdominal or thoracic surgery, continuous infusion of remifentanil (0.05 or 0.1 µg/kg/minute) provided adequate analgesia postoperatively.[388]
|
|
|
|
|
|
|
|
|
|
|
|
|