|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pharmacokinetics and pharmacodynamics of opioids can be influenced by age (also see Chapter 62 ). It is clear that neonates exhibit a reduced rate of elimination of essentially
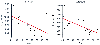 Figure 11-19
Relationship between IC50
and age for fentanyl
and alfentanil. IC50
is the steady-state fentanyl or alfentanil serum
concentration that produces half the maximal spectral edge frequency decrease. (From
Scott JC, Stanski DR: Decreased fentanyl and alfentanil dose requirements with age.
A simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp
Ther 240:159–166, 1987.)
Figure 11-19
Relationship between IC50
and age for fentanyl
and alfentanil. IC50
is the steady-state fentanyl or alfentanil serum
concentration that produces half the maximal spectral edge frequency decrease. (From
Scott JC, Stanski DR: Decreased fentanyl and alfentanil dose requirements with age.
A simultaneous pharmacokinetic and pharmacodynamic evaluation. J Pharmacol Exp
Ther 240:159–166, 1987.)
With advanced age, although pharmacokinetic changes may play a minor role, pharmacodynamic differences are primarily responsible for the decreased dose requirement in the elderly. The concentration of fentanyl and alfentanil necessary to produce half to maximal slowing of the EEG (i.e., the EC50 ) is lower in the elderly compared with younger adults ( Fig. 11-19 ).[334]
Age is inversely correlated with the central volume of distribution, the clearance, and the potency of remifentanil.[335] These combined pharmacokinetic and pharmacodynamic changes mandate a reduction in remifentanil dosage by at least 50% or more in the elderly.
Many opioid pharmacokinetic parameters, especially clearance, appear to be more closely related to lean body mass. This means that opioid dosage regimens may best be based on lean body mass and not total body weight. However, analysis of a large group of patients who received alfentanil suggests that its volume of the central compartment does correlate with total body weight.[336]
Total body weight-based dosing in an obese patient results in much higher remifentanil effect site concentrations than does lean body mass-based dosing.[337] In contrast, for lean patients the concentrations that result from total body weight-based dosing are not much greater than those based on body mass ( Fig. 11-20 ). Clinically, context-sensitive half-times are not significantly different between obese and lean subjects ( Fig. 11-21 ).
There is mounting evidence to suggest that lean body mass is a better predictor of metabolic capacity than total body weight. Ideal body weight, a parameter closely related to lean body mass and one that is perhaps more easily estimated by the clinician, is probably an acceptable alternative.
Renal failure has implications of major clinical importance with respect to morphine and meperidine (also see
 Figure 11-20
A computer simulation of the time course of remifentanil
concentration change, when the dosage regimen is calculated based on the lean body
mass (LBM) or total body weight (TBW) for both obese and lean patients. Note that
TBW-based dosing in an obese patient results in dramatically higher concentrations.
(From Egan TD, Huizinga B, Gupta SK, et al: Remifentanil pharmacokinetics
in obese versus lean patients. Anesthesiology 89:562–573, 1998.)
Figure 11-20
A computer simulation of the time course of remifentanil
concentration change, when the dosage regimen is calculated based on the lean body
mass (LBM) or total body weight (TBW) for both obese and lean patients. Note that
TBW-based dosing in an obese patient results in dramatically higher concentrations.
(From Egan TD, Huizinga B, Gupta SK, et al: Remifentanil pharmacokinetics
in obese versus lean patients. Anesthesiology 89:562–573, 1998.)
Morphine is an opioid with active metabolites that are dependent on renal clearance mechanisms for elimination. Morphine is principally metabolized by conjugation in the liver, and the water-soluble glucuronides (M3G and M6G) are excreted via the kidney. The kidney also plays a role in the conjugation of morphine, accounting for nearly 40% of its metabolism.[298] Patients with renal failure can develop very high levels of M6G and life-threatening respiratory depression ( Fig. 11-22 ). [339] In view of these changes induced by renal failure, morphine may not be a good choice in patients with severely altered renal clearance mechanisms.
The clinical pharmacology of meperidine is also significantly altered by renal failure. Normeperidine, the chief metabolite, has analgesic and CNS excitatory effects.[340] Because
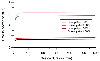 Figure 11-21
A computer simulation of the context-sensitive half-times
(50% decrement times) and 80% decrement times of remifentanil in obese versus lean
subjects. Note that in clinical terms the curves are not grossly different in obese
compared with lean subjects. (From Egan TD, Huizinga B, Gupta SK, et al:
Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology 89:562–573,
1998.)
Figure 11-21
A computer simulation of the context-sensitive half-times
(50% decrement times) and 80% decrement times of remifentanil in obese versus lean
subjects. Note that in clinical terms the curves are not grossly different in obese
compared with lean subjects. (From Egan TD, Huizinga B, Gupta SK, et al:
Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology 89:562–573,
1998.)
 Figure 11-22
Effect of renal failure on pharmacokinetics of morphine.
The graphs show the time-dependent change of the serum concentration of morphine
and its metabolite in patients with normal renal function (top
graph) and patients with renal failure (bottom graph),
who received 0.1 mg/kg morphine IV. (From Osborne R, Joel S, Grebenik K,
et al: The pharmacokinetics of morphine and morphine glucuronides in kidney failure.
Clin Pharmacol Ther 54:158–167, 1993.)
Figure 11-22
Effect of renal failure on pharmacokinetics of morphine.
The graphs show the time-dependent change of the serum concentration of morphine
and its metabolite in patients with normal renal function (top
graph) and patients with renal failure (bottom graph),
who received 0.1 mg/kg morphine IV. (From Osborne R, Joel S, Grebenik K,
et al: The pharmacokinetics of morphine and morphine glucuronides in kidney failure.
Clin Pharmacol Ther 54:158–167, 1993.)
The clinical pharmacology of the fentanyl congeners is not grossly altered by renal failure, although a decrease in plasma protein binding may potentially alter the free fraction of the fentanyl class of opioids.[338] Fentanyl clearance is not altered by renal failure. As with fentanyl, sufentanil pharmacokinetics are not altered in any consistent fashion by renal disease, although greater variability exists in its clearance and elimination half-life when patients have impaired renal function.[341] It has been
Even though the liver is the metabolic organ primarily responsible for opioid biotransformation, the degree of liver failure typically observed in perioperative patients, with the exception of patients undergoing liver transplantation, does not have a major impact on the pharmacokinetics of most opioids (also see Chapter 56 ).
In addition to reduced metabolic capacity (i.e., cytochrome P-450 system and conjugation), liver disease may also lead to reductions in hepatic blood flow, hepatocellular mass, and plasma protein binding. The increase in total body water and the edema of advanced liver disease may alter the distribution characteristics of a drug. Finally, enzyme induction such as observed in early alcoholism can actually increase the liver's metabolic capacity.
Morphine pharmacokinetics are relatively unchanged by liver failure because of the substantial extrahepatic metabolism of morphine. A reduction in hepatic blood flow would be expected to slow the decline of morphine plasma concentrations. Meperidine is an opioid whose pharmacokinetics are altered by liver failure. Hepatic cirrhosis is associated with reduced clearance and a longer terminal half-time of meperidine. The disposition of fentanyl is not altered in patients with cirrhosis during general anesthesia.[343] Reductions in liver blood flow that result from either liver disease or some other disorder (e.g., shock) will delay the decline of fentanyl plasma concentrations. As with meperidine, the pharmacokinetics of alfentanil are also altered by hepatic failure. Ferrier and associates reported that patients with alcoholic cirrhosis have reduced alfentanil plasma clearance and prolonged terminal half-times.[344] Children with cholestatic liver disease who were scheduled to undergo orthotopic liver transplantation showed no changes in alfentanil clearance and volume of distribution at steady state. Although liver blood flow does not affect the kinetics of alfentanil as much as those of morphine and fentanyl, decreases in hepatic blood flow can still decrease alfentanil elimination.[345]
Greater elimination half-lives (3.7 ± 2.6 hours) have also been observed for alfentanil in patients undergoing abdominal aortic surgery. [346] The pharmacokinetics of sufentanil appear to be minimally changed in cirrhotic patients. However, major intra-abdominal surgery can increase the volume of distribution at steady state and the elimination half-life of sufentanil.[312] Remifentanil is an opioid whose pharmacokinetics are completely unchanged by liver disease ( Fig. 11-23 ).[321] Its kinetics do not change during the anhepatic phase of orthotopic liver transplantation.[347] It was reported that 0.25–0.5 µg/kg/minute of remifentanil
 Figure 11-23
Time-dependent changes of blood concentration of remifentanil
in patients with liver disease (top graph) and the
control subjects (bottom graph). In the low-dose
group, remifentanil was infused at 0.0125 µg/kg/minute for 1 hour followed
by 0.025 µg/kg/minute for 3 hours. In the high-dose group, infusion rate of
remifentanil was 0.025 µg/kg/minute for 1 hour followed by 0.05 µg/kg/minutes
for 3 hours. (From Dershwitz M, Hoke JF, Rosow CE, et al: Pharmacokinetics
and pharmacodynamics of remifentanil in volunteer subjects with severe liver disease.
Anesthesiology 84:812–820, 1996.)
Figure 11-23
Time-dependent changes of blood concentration of remifentanil
in patients with liver disease (top graph) and the
control subjects (bottom graph). In the low-dose
group, remifentanil was infused at 0.0125 µg/kg/minute for 1 hour followed
by 0.025 µg/kg/minute for 3 hours. In the high-dose group, infusion rate of
remifentanil was 0.025 µg/kg/minute for 1 hour followed by 0.05 µg/kg/minutes
for 3 hours. (From Dershwitz M, Hoke JF, Rosow CE, et al: Pharmacokinetics
and pharmacodynamics of remifentanil in volunteer subjects with severe liver disease.
Anesthesiology 84:812–820, 1996.)
Cardiopulmonary bypass (CPB) produces significant alterations in the pharmacokinetics of most opioids.[349] These alterations are a result of CPB-induced modifications in distribution volumes (secondary to priming), changes in acid-base balance, organ blood flow, plasma protein concentrations and body temperature. The binding of drugs to components of the bypass circuit can also alter opioid pharmacokinetics (also see Chapter 50 ).
When morphine is given as a premedicant before cardiac anesthesia, its concentrations decline significantly
Acid-base changes can affect a variety of aspects of opioid pharmacokinetics. [356] Respiratory acidosis during
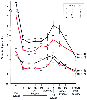 Figure 11-24
Effect of cardiopulmonary bypass on the pharmacokinetics
of sufentanil. Sufentanil was administered as a 30 µg/kg bolus (group I),
a 10 µg/kg/minute infusion (group II), a 20 µg/kg bolus followed by a
0.1 µg/kg/minute infusion (group III), and a 40 µg/kg bolus followed
by a 0.2 µg/kg/minute infusion (group IV). (From Okutani R, Philbin
DM, Rosow CE, et al: Effect of hypothermic hemodilutional cardiopulmonary bypass
on plasma sufentanil and catecholamine concentrations in humans. Anesth Analg 67:667–670,
1988.)
Figure 11-24
Effect of cardiopulmonary bypass on the pharmacokinetics
of sufentanil. Sufentanil was administered as a 30 µg/kg bolus (group I),
a 10 µg/kg/minute infusion (group II), a 20 µg/kg bolus followed by a
0.1 µg/kg/minute infusion (group III), and a 40 µg/kg bolus followed
by a 0.2 µg/kg/minute infusion (group IV). (From Okutani R, Philbin
DM, Rosow CE, et al: Effect of hypothermic hemodilutional cardiopulmonary bypass
on plasma sufentanil and catecholamine concentrations in humans. Anesth Analg 67:667–670,
1988.)
Brain fentanyl levels are higher with respiratory alkalosis. Alkalosis also increases the lipophilicity of several opioids. Alkalosis favors un-ionized morphine and may enhance brain penetration in spite of decreased CBF and increased plasma protein binding. Thus, both intraoperative respiration alkalosis and respiratory acidosis, especially in the immediate postoperative period, can prolong and exacerbate opioid-induced respiratory depression.
It is a common practice to administer reduced doses of opioids to patients suffering from hemorrhagic shock to minimize adverse hemodynamic consequences and to prevent prolonged opioid effect (also see Chapter 63 ). This is at least partly attributable to a pharmacokinetic mechanism. Analysis in studies of pigs receiving fentanyl suggested that central clearance and central- and second-compartment distribution volumes were significantly reduced, resulting in higher fentanyl concentrations for any given dosages and a prolonged context-sensitive half-time ( Fig. 11-25 ).[357] Hemorrhagic shock also altered the pharmacokinetics of remifentanil, suggesting that less remifentanil would be required to maintain a target plasma concentration ( Fig. 11-26 ).[358] However, because of its rapid metabolism, changes in context-sensitive half-time are small.
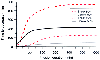 Figure 11-25
A computer simulation of the context-sensitive half-times
(50% decrement times) and 80% decrement times of fentanyl in animals with hemorrhagic
shock and control animals. (From Egan TD, Kuramkote S, Gong G, et al: Fentanyl
pharmacokinetics in hemorrhagic shock: A porcine model. Anesthesiology 91:156–166,
1999.)
Figure 11-25
A computer simulation of the context-sensitive half-times
(50% decrement times) and 80% decrement times of fentanyl in animals with hemorrhagic
shock and control animals. (From Egan TD, Kuramkote S, Gong G, et al: Fentanyl
pharmacokinetics in hemorrhagic shock: A porcine model. Anesthesiology 91:156–166,
1999.)
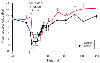 Figure 11-26
Mean spectral edge changes versus time during the remifentanil
infusion. The open and closed circles indicate spectral edge measurements for control
animals and animals with hemorrhagic shock, respectively. (From Johnson
KB, Kern SE, Hamber EA, et al: Influence of hemorrhagic shock on remifentanil:
A pharmacokinetic and pharmacodynamic analysis. Anesthesiology 2001; 94:322–332,
2001.)
Figure 11-26
Mean spectral edge changes versus time during the remifentanil
infusion. The open and closed circles indicate spectral edge measurements for control
animals and animals with hemorrhagic shock, respectively. (From Johnson
KB, Kern SE, Hamber EA, et al: Influence of hemorrhagic shock on remifentanil:
A pharmacokinetic and pharmacodynamic analysis. Anesthesiology 2001; 94:322–332,
2001.)
|
|
|
|
|
|
|
|
|
|
|
|
|