PHARMACOLOGY AND PHARMACODYNAMICS
Developmental Pharmacology
The response of infants and children (and particularly neonates)
to medications is modified by many factors: body composition, protein binding, body
temperature, distribution of cardiac output, functional maturity of the heart, maturation
of the blood-brain barrier, the relative size (as well as functional maturity) of
the liver and kidneys, and the presence or absence of congenital malformations.[23]
[25]
[26]
[27]
[28]
[35]
[36]
[37]
[38]
The body compartments (fat, muscle, water) change with age ( Fig.
60-5
). Total-body water content is significantly
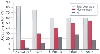 Figure 60-5
Body composition changes rapidly in premature and term
infants during the first 12 months of life. Their high water content provides a
large volume of distribution for water-soluble medications, whereas their low fat
and muscle content provides a small reservoir for drugs that depend on redistribution
into these tissues for termination of drug effects. Thus, body composition may significantly
affect pharmacokinetics and pharmacodynamics. (Data from Friis-Hansen B:
Body composition during growth. In vivo measurements and biochemical data correlated
to differential anatomical growth. Pediatrics 47:169–181, 1971.)
Figure 60-5
Body composition changes rapidly in premature and term
infants during the first 12 months of life. Their high water content provides a
large volume of distribution for water-soluble medications, whereas their low fat
and muscle content provides a small reservoir for drugs that depend on redistribution
into these tissues for termination of drug effects. Thus, body composition may significantly
affect pharmacokinetics and pharmacodynamics. (Data from Friis-Hansen B:
Body composition during growth. In vivo measurements and biochemical data correlated
to differential anatomical growth. Pediatrics 47:169–181, 1971.)
higher in premature than term infants and in term infants than 2-year-olds.[1]
Fat and muscle content increases with age. These alterations in body composition
have several clinical implications for neonates: (1) a drug that is water soluble
has a larger volume of distribution and usually requires a larger initial dose to
achieve the desired blood level (e.g., most antibiotics, succinylcholine); (2) because
neonates have less fat, a drug that depends on redistribution into fat for termination
of its action will have a longer clinical effect (e.g., thiopental); and (3) a drug
that redistributes into muscle may have a longer clinical effect (e.g., fentanyl,
for which, however, saturation of muscle tissue has not been demonstrated).
In addition to these very basic concepts, other important factors
play a role in a neonate's response to medications: (1) delayed excretion because
of the larger volume of distribution, (2) immature hepatic and renal function, and
(3) altered drug excretion caused by lower protein binding. Further perturbations
in drug pharmacodynamics and pharmacokinetics occur with extreme prematurity and
with factors such as sepsis, congestive heart failure, increased intra-abdominal
pressure, controlled ventilation, and poor nutritional status.[25]
[26]
[27]
[28]
[35]
[36]
All these
factors lead to clinically important neonatal patient-to-patient variability in pharmacokinetics
and pharmacodynamics.
Older children tend to have mature renal and hepatic function,
normal adult values for protein, and fat and muscle content approaching adult values.
A greater proportion of cardiac output is diverted to the liver and kidneys—which
also weigh more in relation to body mass—in older children than in infants.
These factors usually mean that most medications have a shorter half-life in children
older than 2 years than in adults. As the child approaches adulthood, the half-life
of many drugs lengthens. In general, most medications will have a prolonged elimination
half-life in premature and term infants, a shortened half-life in children older
than 2 years up to
the early teen years, and a lengthening of half-life in those approaching adulthood.
[36]
 Figure 60-5
Body composition changes rapidly in premature and term
infants during the first 12 months of life. Their high water content provides a
large volume of distribution for water-soluble medications, whereas their low fat
and muscle content provides a small reservoir for drugs that depend on redistribution
into these tissues for termination of drug effects. Thus, body composition may significantly
affect pharmacokinetics and pharmacodynamics. (Data from Friis-Hansen B:
Body composition during growth. In vivo measurements and biochemical data correlated
to differential anatomical growth. Pediatrics 47:169–181, 1971.)
Figure 60-5
Body composition changes rapidly in premature and term
infants during the first 12 months of life. Their high water content provides a
large volume of distribution for water-soluble medications, whereas their low fat
and muscle content provides a small reservoir for drugs that depend on redistribution
into these tissues for termination of drug effects. Thus, body composition may significantly
affect pharmacokinetics and pharmacodynamics. (Data from Friis-Hansen B:
Body composition during growth. In vivo measurements and biochemical data correlated
to differential anatomical growth. Pediatrics 47:169–181, 1971.)